The only infusion set indicated for up to 7 days of wear
The Medtronic Extended™ infusion set and Extended™ reservoir designed for life with fewer interruptions
One set change per week
compared to every 2 to 3 days1
The first and only infusion set and reservoir to be designed for longer wear, without compromising comfort1 or insulin delivery.2
5 changes per month versus 10 changes per month with another pump system or infusion set.
Advancing insulin delivery in pump therapy
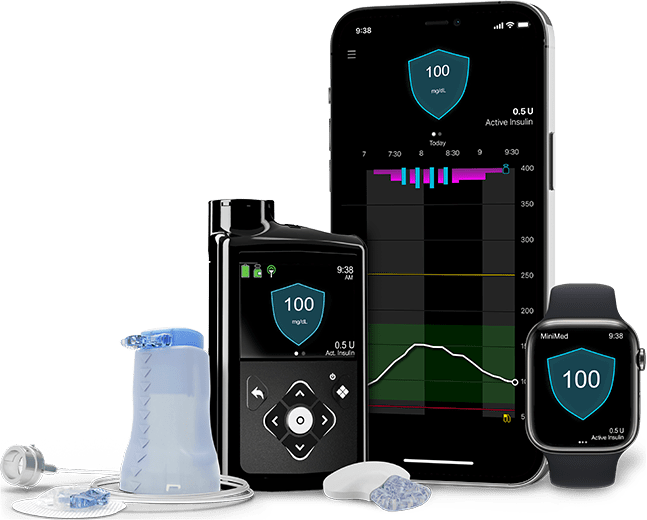
MiniMed™ 780G system
Use the Medtronic Extended™ infusion set and Extended™ reservoir with the MiniMed™ 780G system and other Medtronic insulin pumps.
The MiniMed™ 780G system is the only system with Meal Detection™ technology† that provides automatic adjustments and corrections to sugar levels every 5 minutes.‡ Learn more about the MiniMed™ 780G system.
Request info
Ready, Extended™ Infusion Set, Go!
Jaylen's story

Simple to use and virtually painless5
With the all-in-one serter, set changes are quick and easy to do. Watch how to insert the Extended™ infusion set and reservoir.




To get the first and only infusion set designed for twice the wear, order online or by calling 1-800-646-4633 and select option 2. (Monday - Friday, 8:00 a.m. to 6:00 p.m. CT). Please note that the Extended™ infusion set requires a new prescription.
Frequently asked questions
No, other Medtronic infusion sets are only cleared for 2-3 days. The Extended™ infusion set has new technology and different materials that allow it to be worn for twice the wear time.
No, the standard reservoir is labeled for use up to 3 days and must be changed every 3 days. We recommend using the Medtronic Extended™ infusion set and Extended™ reservoir together. The Extended™ reservoir is labeled for use up to 7 days and thus only needs to be changed when insulin runs out or when the infusion set is changed.
References
1. Chattaraj S, et al. The Medtronic extended-wear infusion set: Determining mechanisms of action. As presented at the 14th International Conference on Advanced Technologies & Treatments for Diabetes; June 2-5, 2021.
2. Chattaraj S, et al. Study of insulin stability impact on pump therapy: Test model development. Diabetes. 2020;69 (Supplement 1):1012-P
3. Zhang G, et al. Assessment of adhesive patches for an extended-wear infusion set. Diabetes. 2020;69 (Supplement 1):986-P
4. Chattaraj S, et al. 1167-P - CSII and Insulin: Does extending the wear duration of infusion sets save expensive insulin? 80th ADA International Conference, June 2020.
5. Medtronic Data on File. MiniMed™ Mio™ Advance US Pivotal Trials. 152 patients interviewed. 2020.
6. MRM 17430 MiniMed™ Mio Advance claims from Human Factors Testing. Medtronic data on file.
7. IFU & User Guide Comparison. Medtronic data on File.
* vs the 3-days infusion set MiniMed™ Quick-set™
† Taking a bolus 15 – 20 minutes before a meal helps to keep blood sugar levels under control after eating.
‡ Refers to SmartGuard™ feature. Individual results may vary.
Important Safety Information
Medtronic Extended™ infusion set
The Extended™ Infusion Set is indicated for up to 7 days of wear for the subcutaneous infusion of insulin from an infusion pump. It is NOT indicated for intravenous (IV) infusion or the infusion of blood or blood products. Inaccurate medication delivery, infection and/or site irritation may result from improper insertion and maintenance of the infusion site. Before insertion, clean the insertion site with isopropyl alcohol. Remove the needle guard before inserting the infusion set. If using this infusion set for the first time, do the first set-up in the presence of your healthcare professional. Do not leave air in the infusion set. Prime completely. Check frequently to make sure the soft cannula remains firmly in place as you may not feel pain if it pulls out. The soft cannula must always be completely inserted to receive the full amount of medication. If the infusion site becomes inflamed, replace the set, and use a new site until the first site has healed. Replace the infusion set if the tape becomes loose, or if the soft cannula becomes fully or partially dislodged from the skin. Regularly replace the infusion set as indicated in the instructions for use, or per the insulin labeling, whichever duration is shorter. For more details, see https://www.medtronicdiabetes.com/important-safety-information.
Medtronic Extended™ reservoir
The Extended™ Reservoir is indicated for the subcutaneous infusion of insulin from compatible Medtronic insulin pumps and infusion sets. Refer to your Medtronic insulin pump user guide for a list of compatible insulins and infusion sets. The reservoir is contraindicated for the infusion of blood or blood products. Use for a maximum of 7 days, or according to the insulin labeling, whichever duration is shorter. For more details, see
important safety information.
MiniMed™ 770G System With SmartGuard™ Technology
The MiniMed™ 770G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons two years of age and older requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 770G System includes SmartGuard™ technology, which can be programmed to automatically adjust delivery of basal insulin based on continuous glucose monitoring (CGM) sensor glucose values (SG) and can suspend delivery of insulin when the SG value falls below or is predicted to fall below predefined threshold values.
The Medtronic MiniMed™ 770G System consists of the following devices: MiniMed™ 770G Insulin Pump, the Guardian™ Link (3) Transmitter, the Guardian™ Sensor (3), one-press serter, the Accu-Chek® Guide Link blood glucose meter, and the AccuChek®Guide Test Strips. The system requires a prescription.
The Guardian™ Sensor (3) has not been evaluated and is not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. All therapy adjustments should be based on measurements obtained using a blood glucose meter and not on values provided by the Guardian™ Sensor (3).
All therapy adjustments should be based on measurements obtained using the Accu-Chek® Guide Link blood glucose meter and not on values provided by the Guardian™ Sensor (3). Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the Accu-Chek® Guide Link blood glucose meter. Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an alternative site. It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise.
WARNING: Do not use the SmartGuard™ Auto Mode for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in SmartGuard™ Auto Mode.
WARNING: Do not use the MiniMed™ 770G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 770G system.
Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. The safety of the MiniMed™ 770G system has not been studied in pregnant women. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult https://www.medtronicdiabetes.com/important-safetyinformation#minimed-770g and the appropriate user guide at https://www.medtronicdiabetes.com/download-library
MiniMed™ 780G system with SmartGuard™ technology with Guardian™ 4 sensor
The MiniMed™ 780G system is intended for continuous delivery of basal insulin at selectable rates, and the administration of insulin boluses at selectable amounts for the management of type 1 diabetes mellitus in persons seven years of age and older requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 780G system includes SmartGuard™ technology, which can be programmed to automatically adjust insulin delivery based on the continuous glucose monitoring (CGM) sensor glucose values and can suspend delivery of insulin when the sensor glucose (SG) value falls below or is predicted to fall below predefined threshold values.
The Medtronic MiniMed™ 780G system consists of the following devices: MiniMed™ 780G insulin pump, the Guardian™ 4 transmitter, the Guardian™ 4 sensor, One-press serter, the Accu-Chek™ Guide Link blood glucose meter, and the Accu-Chek™ Guide test strips. The system requires a prescription from a healthcare professional.
The Guardian™ 4 sensor is intended for use with the MiniMed™ 780G system and the Guardian 4 transmitter to monitor glucose levels for the management of diabetes. The sensor is intended for single use and requires a prescription. The Guardian™ 4 sensor is indicated for up to seven days of continuous use.
The Guardian™ 4 sensor is not intended to be used directly to make therapy adjustments while the MiniMed™ 780G is operating in manual mode. All therapy adjustments in manual mode should be based on measurements obtained using a blood glucose meter and not on values provided by the Guardian™ 4 sensor. The Guardian™ 4 sensor has been studied and is approved for use in patients ages 7 years and older and in the arm insertion site only. Do not use the Guardian™ 4 sensor in the abdomen or other body sites including the buttocks, due to unknown or different performance that could result in hypoglycemia or hyperglycemia.
WARNING: Do not use the SmartGuard™ feature for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in the SmartGuard™ feature.
WARNING: Do not use the MiniMed™ 780G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 780G system.
WARNING: Do not use SG values to make treatment decisions, including delivering a bolus, while the pump is in Manual Mode. When the SmartGuard™ feature is active and you are no longer in Manual Mode, the pump uses an SG value, when available, to calculate a bolus amount. However, if your symptoms do not match the SG value, use a BG meter to confirm the SG value. Failure to confirm glucose levels when your symptoms do not match the SG value can result in the infusion of too much or too little insulin, which may cause hypoglycemia or hyperglycemia.
Pump therapy is not recommended for people whose vision or hearing does not allow for the recognition of pump signals, alerts, or alarms. The safety of the MiniMed™ 780G system has not been studied in pregnant women, persons with type 2 diabetes, or in persons using other anti-hyperglycemic therapies that do not include insulin. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult https://www.medtronicdiabetes.com/important-safety-information#minimed-780g and the appropriate user guide at https://www.medtronicdiabetes.com/download-library