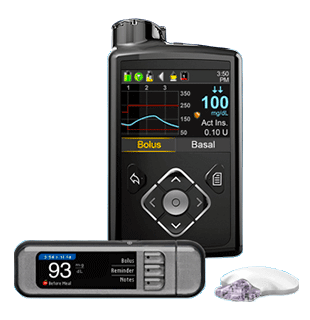
CONTOUR®NEXT LINK meters
The only FDA approved linking meters for use with Medtronic MiniMed™ 670G and MiniMed™ 630G pump systems.
The meters from CONTOUR®NEXT LINK** were exclusively designed to transmit proven highly accurate1,2 CONTOUR®NEXT blood glucose test strip results wirelessly to compatible MiniMed™ insulin pumps for easy and accurate CGM calibration and optimal insulin dosing.*
To purchase CONTOUR®NEXT test strips, call 800-646-4633, option 2 or visit Diabetes.shop.
The CONTOUR®NEXT LINK 2.4 Meter from Ascensia Diabetes Care is the only approved linking meter for use with the MiniMed™ 670G and MiniMed™ 630G systems with SmartGuard™ technology.
Note: With the advancement of the MiniMed™ 780G system, Medtronic has decided to simplify insulin pump offerings and discontinue sales of the MiniMed™ 600 series.
Accuracy
- The high accuracy and precision demonstrated by the CONTOUR®NEXT LINK 2.4 Meter has helped to close the gap between laboratory accuracy and real-word test results.2
- Clinical studies have demonstrated that accurate meter readings are important for insulin pump systems to help avoid hypoglycemia, hyperglycemia, and insulin dosing errors.3
Convenience and simplicity
- Sends results proven to be highly accurate2 to the MiniMed™ 670G and MiniMed™ 630G pumps for easy insulin dosing and CGM calibration.2
CONTOUR®NEXT test strips
Small sample size
Only 0.6 μl required.
No coding™ technology
That means one less step for you.
Second-chance® sampling
Allows you to reapply blood to the same test strip - which may help you save money.
Act now and order your CONTOUR®NEXT test strips by calling 800-646-4633, option 2 or by visiting Diabetes.shop.
Ascensia, the Ascensia Diabetes Care logo, Contour, No Coding, and Second-Chance are trademarks and/or registered trademarks of Ascensia Diabetes Care.
PP-CN-LNK-US-0485
*Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an alternative site (palm) or from a control solution test. Do not calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise.
**The CONTOUR®NEXT LINK Meter is used with the MiniMed™ 530G system. The CONTOUR®NEXT LINK 2.4 Meter is used with the MiniMed™ 630G system and the MiniMed™ 670G system.
References
1. Bernstein, et al. A New Test Strip Technology Platform for Self-Monitoring of Blood Glucose. J Diabetes Sci Technol 2013; 7(5): 1386-1399.2. Bailey J, Wallace J, Greene C. Accuracy and User Performance Evaluation of the CONTOUR®NEXT LINK 2.4 Blood Glucose Monitoring System. Clinica Chimica Acta 448 (2015) 139 -145.
3. Walsh, et al. New Criteria for Assessing the Accuracy of Blood Glucose Monitors Meeting. Diabetes Science & Technology, 2012 6(2): 466-474.
Important Safety Information: MiniMed™ 670G System
The Medtronic MiniMed™ 670G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons, fourteen years of age and older, requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 670G system includes SmartGuard technology, which can be programmed to automatically adjust delivery of basal insulin based on continuous glucose monitor sensor glucose values, and can suspend delivery of insulin when the sensor glucose value falls below or is predicted to fall below predefined threshold values. The system requires a prescription. The Guardian Sensor (3) glucose values are not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. A confirmatory finger stick test via the CONTOUR®NEXT LINK 2.4 blood glucose meter is required prior to making adjustments to diabetes therapy. All therapy adjustments should be based on measurements obtained using the CONTOUR®NEXT LINK 2.4 blood glucose meter and not on values provided by the Guardian Sensor (3). Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the CONTOUR®NEXT LINK 2.4 blood glucose meter. Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an alternative site (palm) or from a control solution test. Do not calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise. If a control solution test is out of range, please note that the result may be transmitted to your pump when in the “Always” send mode.
WARNING: Medtronic performed an evaluation of the MiniMed™ 670G system and determined that it may not be safe for use in children under the age of 7 because of the way that the system is designed and the daily insulin requirements. Therefore this device should not be used in anyone under the age of 7 years old. This device should also not be used in patients who require less than a total daily insulin dose of 8 units per day because the device requires a minimum of 8 units per day to operate safely.
Only use rapid acting U100 insulin with this system. Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. The safety of the MiniMed™ 670G system has not been studied in pregnant women. For complete details, including product and important safety information concerning the system and its components, please consult http://www.medtronicdiabetes.com/important-safety-information and the appropriate user guide at http://www.medtronicdiabetes.com/download-library.
Important Safety Information: MiniMed™ 630G System with SmartGuard™ Technology
Indicated for the continuous delivery of insulin, at set and variable rates, for the management of diabetes mellitus. MiniMed™ 630G system is approved for ages 14 years or older with Guardian™ Sensor 3 and MiniMed™ 630G system is approved for ages 16 years or older with Enlite™ sensor. Both systems require a prescription. Insulin infusion pumps and associated components of insulin infusion systems are limited to sale by or on the order of a physician and should only be used under the direction of a healthcare professional familiar with the risks of insulin pump therapy. Pump therapy is not recommended for people who are unwilling or unable to perform a minimum of four blood glucose tests per day. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Insulin pumps use rapid-acting insulin. If your insulin delivery is interrupted for any reason, you must be prepared to replace the missed insulin immediately. Replace the infusion set every 48–72 hours, or more frequently per your healthcare professional’s instructions. Insertion of a glucose sensor may cause bleeding or irritation at the insertion site. Consult a physician immediately if you experience significant pain or if you suspect that the site is infected. The information provided by CGM systems is intended to supplement, not replace, blood glucose information obtained using a blood glucose meter. A confirmatory fingerstick using a CONTOUR®NEXT LINK 2.4 meter is required prior to making adjustments to diabetes therapy. Always check the pump display when using a CONTOUR®NEXT LINK 2.4 meter, to ensure the glucose result shown agrees with the glucose results shown on the meter. Do not calibrate your CGM device or calculate a bolus using a result taken from an Alternative Site (palm) or a result from a control solution test. If a control solution test is out of range, please note that the result may be transmitted to your pump when in the “Always” send mode. It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise. The MiniMed™ 630G system is not intended to be used directly for preventing or treating hypoglycemia but to suspend insulin delivery when the user is unable to respond to the Suspend on low alarm and take measures to prevent or treat hypoglycemia themselves. Therapy to prevent or treat hypoglycemia should be administered according to the recommendations of the user’s healthcare provider.
WARNING: The SmartGuard™ Suspend on low feature will cause the pump to temporarily suspend insulin delivery for two hours when the sensor glucose reaches a set threshold. Under some conditions of use the pump can suspend again, resulting in very limited insulin delivery. Prolonged suspension can increase the risk of serious hyperglycemia, ketosis, and ketoacidosis. Before using the SmartGuard™ feature, it is important to read the SmartGuard™ feature information in the User Guide and discuss proper use of the feature with your healthcare provider.
See www.medtronicdiabetes.com/important-safety-information and the appropriate user guides for additional important details.

Insertion of a glucose sensor may cause bleeding or irritation at the insertion site. Consult a physician immediately if you experience significant pain or if you suspect that the site is infected. Please visit www.medtronicdiabetes.com/important-safety-information for more details.
The CONTOUR®NEXT LINK 2.4 Meter is used with the MiniMed™ 630G system.
Ascensia, the Ascensia Diabetes Care logo and Contour are trademarks and/or registered trademarks of Ascensia Diabetes Care.
MiniMed™ is a registered trademark and SmartGuard is a trademark of Medtronic, MiniMed™ Inc.