MEDICAL DEVICE CORRECTION
InPen™ smart insulin pen Cartridge Holder Assembly Issue
| Model/CFN | Impacted lot numbers |
|---|---|
| MMT-105NNBLNA | D0020, D0034, D0036, D0038, D0045, D0046, D0050, D0057 |
| MMT-105NNGYNA | D0026, D0037, D0039, D0041, D0042, D0043, D0049 |
| MMT-105NNPKNA | D0029, D0030, D0040, D0051, D0062 |
| MMT-105ELBLNA | D0021, D0033, D0035, D0044, D0059, D0060, D0061 |
| MMT-105ELGYNA | D0031, D0032, D0047, D0068 |
| MMT-105ELPKNA | D0027, D0028, D0048 |
March 2025
Medtronic is contacting you with a medical device correction regarding InPen smart insulin pen. Some InPens have been identified with an issue that can cause difficulty in removing the cartridge holder or installing the insulin cartridge into the cartridge holder.
Issue Description:
Some InPens from certain lots may have been incorrectly assembled. For affected InPens, users could potentially experience either one of two issues: either the insulin cartridge will not fit into the cartridge holder; or the cartridge holder may be difficult to remove from the insulin pen, requiring a significant amount of force.
Risk to Health:
An InPen with an insulin cartridge that will not fit into the cartridge holder cannot be used. An InPen with a cartridge holder that is difficult to remove should not be used even if you are able to do so. If you do not have backup insulin when you need it, you may experience temporary hyperglycemia.
Between May 2024 and November 2024, Medtronic received 31 complaints potentially related to this issue. There have not been any serious adverse events associated with these complaints.
Actions Required:
If you have already inserted your insulin cartridge into your InPen and are using your InPen without difficulty removing the cartridge holder, then your InPen is not affected by this issue, and you can continue to use your InPen as normal.
Locate the lot number for your InPen. You can find the lot number in the two locations indicated in the sample images below.
The first location is the fold-over label that is affixed to the InPen box/carton The second location is on the dose knob portion of the InPen 
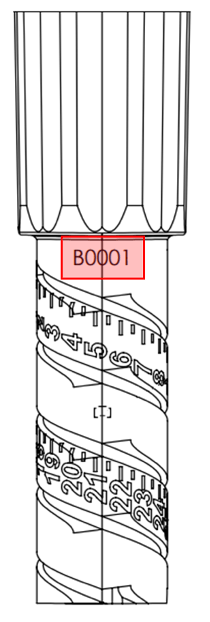
Verify if your lot number matches one of the impacted lot numbers listed at the top of this letter.
If you have an InPen from one of the affected lot numbers listed at the top of this letter and have not yet started using it or have had difficulty in removing the cartridge holder or inserting the insulin cartridge, please call 1-800-646-4633, option 1, or go to https://info.medtronicdiabetes.com/ICH-replacement-form to request a replacement. Do not use this InPen and dispose of it according to local regulations.
Regardless if your InPen is affected, please acknowledge that you have read and understood this notification and have followed the actions listed in this letter by either completing and returning the confirmation form, scanning the QR code below with your phone or tablet, or by visiting https://info.medtronicdiabetes.com/cartridge-holder

Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail, or by fax.
- Complete and submit the report online: www.fda.gov/MedWatch/report.htm.
- Regular mail or fax: Download the form at www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178
As always, we are here to support you and ensure we are delivering the highest quality products possible. If you have further questions or need assistance, please call the Medtronic 24-Hour Technical Support line at 1-800-646-4633, option 1.
Sincerely,
Julio Salwen
Vice President, Quality
Medtronic Diabetes
Frequently asked questions
Yes. If you have a pen from one of the impacted lots that you have not yet started using or have not been able to use successfully, please call 1-800-646-4633, option 1 or visit https://info.medtronicdiabetes.com/ICH-replacement-form to request a replacement. Do not use this InPen and dispose of it according to local regulations.
You received this notification because you were identified as a customer with an InPen from a lot that may be impacted by a manufacturing issue.
Yes, it is important to acknowledge the safety notice. Please follow the instructions provided in the notice. Patients can click the button in the email or text message stating, "I have read and understood the actions required by me in this Medical Device Correction" This acknowledgment ensures that you are aware of the critical safety information and allows us to provide you with the necessary support. We will continue to send messages until you acknowledge that you have understand the actions required by you.
If your InPen is functioning normally, you should continue to use it as instructed by your healthcare professional. If you have not started using an InPen from an impacted lot, please contact Medtronic to request a replacement.