SAFETY ALERT
MiniMed™ 600 series and 700 series pump systems — battery status alerts and alarms
Products: All MiniMed™ 600 series and MiniMed™ 700 series insulin pump models, including MiniMed™ 630G, MiniMed™ 670G, MiniMed™ 770G, and MiniMed™ 780G
July 2024
Dear Valued Customer:
Medtronic is contacting you with a reminder about the importance of following your pump’s built-in alerts and alarms for battery status when they are displayed on the pump, as outlined in the instructions for use. The MiniMed™ 600 series and 700 series pump systems are designed to monitor the pump’s battery life over time and will generate a series of visual, audible, and vibratory low battery alerts and alarms to remind you when it is time to replace the battery.
- The “Low Battery Pump” alert will display when your pump has up to 10 hours of battery life left.
- The “Replace Battery” alert will display when the pump has less than 30 minutes of battery life left.
If the battery is not replaced within 10 minutes, a siren will sound and repeat once every minute until the “Replace Battery Now” alarm is displayed. At that time, the pump will stop insulin delivery.
If the battery is not replaced within 10 minutes of this alarm, a siren will sound, and the pump will shut down.
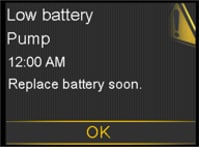
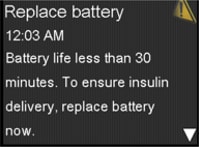
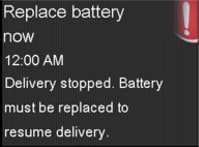 Visual Pump Notifications before Battery Depletion
Visual Pump Notifications before Battery DepletionIn addition, the pump displays the battery status on the home screen.
Example of Battery Icon on Pump Home Screen Status Bar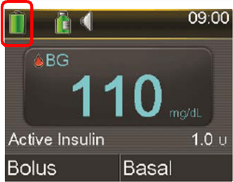
Issue Description:
We have found that in some instances, pumps that have been dropped, bumped, or experienced physical impact, may have damage to internal electrical components, which may cause reduced battery life on the pump. Please note that even a single drop could result in reduced battery life, either immediately after the drop, or over time. Your pump will still generate low battery and replace battery alerts and alarms; however, these notifications may display sooner than expected, resulting in the battery needing to be replaced sooner than expected. No serious injuries have been confirmed to be related to the battery depleting sooner than expected on MiniMed™ 600 and 700 series pumps.
Recommended Actions for Customers:
- Always pay attention to alerts and alarms displayed on your pump.
- Be aware that you can check your pump’s battery level anytime on the status bar located on the pump home screen.
- If your pump has been dropped, bumped, or has experienced physical impact, pay special attention to any pump alerts and alarms, including low battery alerts, as they may occur earlier than expected. Follow any prompts on the pump to replace the battery. Refer to your pump’s user guide for instructions on how to replace the battery. If you notice any significant changes in battery life or need additional troubleshooting assistance, please contact the Medtronic 24-Hour Technical Support line for further help.
- Ensure you always have extra new AA lithium or alkaline batteries or fully charged NiMH batteries available, along with your other emergency kit supplies.
- As indicated in the pump’s user guide, keep an emergency kit available at all times to confirm that necessary supplies are ready.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report online: www.fda.gov/MedWatch/report.htm.
- Regular mail or fax: Download format www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
Please acknowledge that you have read and understood this notification and have followed the actions listed in this letter by either completing and returning the confirmation form, scanning the QR code below, or visiting https://info.medtronicdiabetes.com/battery-status-acknowledgement to acknowledge this communication.

To scan the QR code open the built-in camera app on your phone or tablet. Point the camera at the QR code. Tap the banner that appears on your phone or tablet and the instructions on the screen to finish.
As always, we are here to support you. If you have further questions or need assistance, please call the Medtronic 24-Hour Technical Support line at 800-646-4633, option 1.
Sincerely,
Julio Salwen
Vice President, Quality
Medtronic Diabetes
Frequently asked questions
Yes, by definition, this is a recall however it does not mean that you stop using the product or return it to the company. In this case, this is a safety alert/reminder for patients to closely monitor their battery life for the pump. This specifically applies if a pump has been dropped, bumped, or experienced physical impact, which may cause reduced battery life. Please note that even a single drop could result in reduced battery life, either immediately after the drop, or over time. (https://www.fda.gov/medical-devices/medical-device-recalls/what-medical-device-recall).
You received this notification because you were identified as a customer with a MiniMed™ 600 series or MiniMed™ 700 series insulin pump.
Thank you for reaching out to us. We are still in the process of notifying all impacted pump users. You can also visit our notice page at https://www.medtronicdiabetes.com/Battery to review the details about this issue.
Yes, it is important to acknowledge the safety notice. Please follow the instructions provided in the notice. U.S. patients and all other regions where confirmation is required can sign and return the confirmation letter they received in the mail, or they can click the button in the email stating, "I have read and understand the actions required by me in the Safety Alert." This acknowledgment ensures that you are aware of the critical safety information and allows us to provide you with the necessary support.
Yes, you can still use your insulin pump as normal. Always pay attention to alerts and alarms displayed on your pump. This notice specifically applies to users whose pumps have been dropped, bumped, or experienced physical impact, which may cause reduced battery life. If your pump has been subjected to such impacts, please be extra vigilant in monitoring your pump’s battery status and follow all prompts accordingly.
Your device does not require replacement, please use your insulin pump as normal and ensure you always pay attention to alerts and alarms displayed on your pump.
If you notice any significant changes in battery life on your pump or need additional troubleshooting assistance, please contact Medtronic 24-Hour Technical Support at 1-800-646-4633.
If you are unsure about your battery's performance, closely monitor the battery status on your pump's home screen and follow all built-in alerts and alarms for battery status. If you have any concerns or uncertainties, please contact Medtronic's customer support for further troubleshooting and assistance.