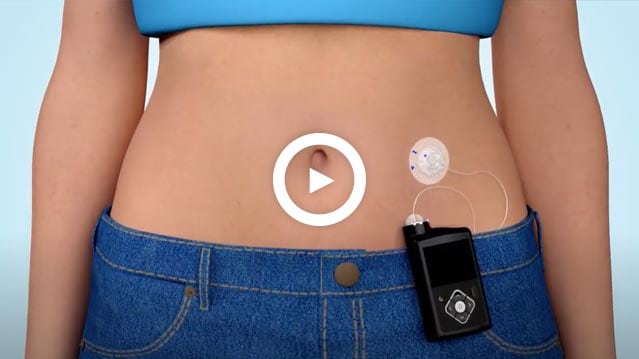
Kids play more. You worry less.
Thousands of children are diagnosed with type 1 diabetes every year.1 Understanding a new diagnosis can be overwhelming. It’s tough to know what’s next for your child and family. We want to help. Here, you can learn about diabetes and therapy options, and get tips to help make life easier.
Lenny™

Common questions
Here are some of the most common questions that families have following a diabetes diagnosis.
Get answers

Community resources
Want to learn more? There are many great resources created by other organizations within the diabetes community.
Resources

Therapy & technology overview
There are many ways to successfully manage diabetes. This section helps explain how individuals can manage diabetes and some of the latest technology options available today.
Videos

Therapy & technology quiz
Do you understand the different options available to manage insulin-dependent diabetes? Here are a few questions to see how well you’ve mastered the information.
Quiz

How to get started on pump therapy
Ready to start technology? One of the first steps is talking to your child's healthcare professional. You can use this resource to guide the conversation.
Download

Parenting a teen with diabetes
Parenting a teenager isn’t easy, especially when you add a new diabetes diagnosis into the mix. Here are some common questions parents have regarding diabetes in the teenage years.
Learn more

Sleepover guide
Aside from their jammies, pillow, and favorite blanket or stuffed animal, kids with diabetes have to pack a bit more to ensure a safe and fun sleepover! Here are recommended items your child may want to take when heading over to a friend’s house.

Coach, babysitter, and teacher guides
Leaving your child with a coach, babysitter, or teacher for the first time can be a bit nerve-wracking. However, with the right tools and knowledge, your child can participate in activities just like anyone else. Here are a few things to keep in mind.
Explore your options
To receive informative emails and speak with a trained diabetes therapy consultant, fill out the form to be contacted within 5 business days or call 1-800-646-4633 (M–F, 8AM–6PM CT).
Lenny can’t wait to go to summer camp!
Check out Lenny’s scavenger hunt. Download for your kids today.

Get pumped with Lenny!
Check out Lenny's coloring sheets.
Available to download for your kids!
References
1 CDC National Diabetes Statistics Report, 2020
* Refers to Auto Mode. Some user interaction required. Individual results may vary.
Actor portrayal used on this webpage.
Important Safety Information: MiniMed™ 770G System With SmartGuard™ Technology
The MiniMed™ 770G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons two years of age and older requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 770G System includes SmartGuard™ technology, which can be programmed to automatically adjust delivery of basal insulin based on continuous glucose monitoring (CGM) sensor glucose values (SG) and can suspend delivery of insulin when the SG value falls below or is predicted to fall below predefined threshold values.
The Medtronic MiniMed™ 770G System consists of the following devices: MiniMed™ 770G Insulin Pump, the Guardian™ Link (3) Transmitter, the Guardian™ Sensor (3), one-press serter, the Accu-Chek® Guide Link blood glucose meter, and the Accu-Chek® Guide Test Strips. The system requires a prescription.
The Guardian™ Sensor (3) has not been evaluated and is not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. All therapy adjustments should be based on measurements obtained using a blood glucose meter and not on values provided by the Guardian™ Sensor (3).
All therapy adjustments should be based on measurements obtained using the Accu-Chek® Guide Link blood glucose meter and not on values provided by the Guardian™ Sensor (3). Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the Accu-Chek® Guide Link blood glucose meter. Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an alternative site. It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise.
WARNING: Do not use the SmartGuard™ Auto Mode for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in SmartGuard™ Auto Mode.
WARNING: Do not use the MiniMed™ 770G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 770G system.
Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. The safety of the MiniMed™ 770G system has not been studied in pregnant women. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult https://www.medtronicdiabetes.com/important-safety-information#minimed-770g and the appropriate user guide at http://www.medtronicdiabetes.com/download-library
Important Safety Information: MiniMed™ 670G System
The Medtronic MiniMed™ 670G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons, seven years of age and older, requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 670G system includes SmartGuard™ technology, which can be programmed to automatically adjust delivery of basal insulin based on Continuous Glucose Monitor sensor glucose values and can suspend delivery of insulin when the sensor glucose value falls below or is predicted to fall below predefined threshold values. The system requires a prescription. The Guardian™ Sensor (3) glucose values are not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. A confirmatory finger stick test via the CONTOUR®NEXT LINK 2.4 blood glucose meter is required prior to making adjustments to diabetes therapy. All therapy adjustments should be based on measurements obtained using the CONTOUR®NEXT LINK 2.4 blood glucose meter and not on values provided by the Guardian™ Sensor (3). Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the CONTOUR®NEXT LINK 2.4 blood glucose meter. Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an Alternative Site (palm) or from a control solution test. It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise. If a control solution test is out of range, please note that the result may be transmitted to your pump when in the “Always” send mode.
WARNING: Medtronic performed an evaluation of the MiniMed™ 670G system and determined that it may not be safe for use in children under the age of 7 because of the way that the system is designed and the daily insulin requirements. Therefore this device should not be used in anyone under the age of 7 years old. This device should also not be used in patients who require less than a total daily insulin dose of 8 units per day because the device requires a minimum of 8 units per day to operate safely.
Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. The safety of the MiniMed™ 670G system has not been studied in pregnant women. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult http://www.medtronicdiabetes.com/important-safety-information#minimed-670g and the appropriate user guide at http://www.medtronicdiabetes.com/download-library