URGENT MEDICAL DEVICE RECALL INFORMATION
Medtronic MiniMed™ infusion sets - Potential over-delivery of insulin
September 11, 2017
Because the safety of our customers is our top priority, we are voluntarily recalling specific lots of infusion sets used with Medtronic insulin pumps.
Explanation of the Issue
Medtronic has become aware of recent reports of potential over-delivery of insulin shortly after an infusion set change. Over-delivery of insulin can cause hypoglycemia and in extreme cases, death. Medtronic has received reports of hypoglycemia requiring medical intervention potentially related to this issue.
Our investigation has shown this can be caused by fluid blocking the infusion set membrane during the priming/fill-tubing process. A membrane blocked by fluid most likely occurs if insulin, alcohol, or water is spilled on top of the insulin reservoir which then could prevent the infusion set from working properly. Infusion sets currently being shipped by Medtronic contain a new and enhanced membrane material that significantly reduces this risk.
Actions Required by You
- Go to https://checklots.medtronicdiabetes.com to determine if you have recalled infusion sets. You will be prompted to enter the REF and LOT numbers for all infusion sets in your possession. The website will then tell you which infusion sets are part of this recall and which are not.
Your REF and LOT numbers are listed on the labels as shown in the examples below:
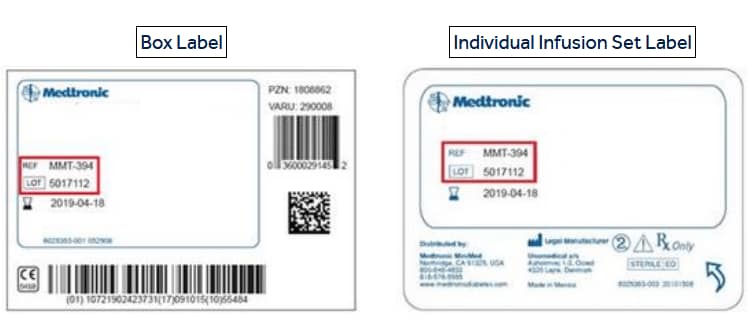
- Medtronic recommends you not use recalled infusion sets.
- If you have new and enhanced infusion sets that are not part of this recall, use only those sets starting with your next set change. As a reminder, we have Key Steps regarding the priming/fill-tubing process.
- If you only have recalled infusion sets right now, it is very important that you carefully follow the Key Steps.
- Return your recalled infusion sets within the next four weeks the pre-paid label that has been sent to you. Medtronic will replace the recalled infusion sets free of charge. For detailed information on returns, see the Return Instructions.
What if I have more questions?
Follow the process on the website at https://checklots.medtronicdiabetes.com. If you have additional questions, please refer to the Frequently Asked Questions or call Medtronic at 1.888.204.7616.
You can also report an adverse event to the FDA’s MedWatch Adverse Event Reporting program:
- Online at: http://www.fda.gov/safety/medwatch/howtoreport/default.htm
- Report by telephone: 1.800.FDA.1088
- Fax report: 1.800.FDA.0178
Medtronic considers patient safety and customer satisfaction our top priorities. We appreciate your time and attention in reading this important notification.
Sincerely
James Dabbs
Vice President, Quality Assurance
Medtronic Diabetes
Frequently asked questions
Yes, this is a voluntary recall because your safety is our top priority. We are notifying all customers along with their corresponding healthcare providers. You can check to see if your infusion sets are part of the recall by visiting https://www.medtronicdiabetes.com.
Medtronic recommends that customers do not use and return any recalled infusion sets they have. Medtronic will replace the recalled infusion sets free of charge. Please be aware that the exchange process will take some time, as we need to build the necessary inventory while ensuring supply for ongoing use.
We recommend that you do not use your recalled infusion sets and use only new and enhanced sets starting with your next infusion set change.
If you only have recalled infusion sets, it is very important to carefully follow the instructions within the Key Steps regarding the priming/fill-tubing process.
If you do not have new and enhanced infusion sets, you will need to speak with your healthcare provider to discuss your alternative therapy options. Please know that we are making this recommendation for your safety.
It is essential that you continue to order your infusion sets as you usually do, even if you are expecting replacement infusion sets. If you have a current supply order, do not cancel it.
Please note that replacement sets may take longer to receive than your normal shipments. You may not receive all your replacements at once. All infusion sets you receive moving forward will be new and enhanced.
Important information about how to prime your infusion set properly can be found in the Key Steps instructions included in your recall notification. In addition, Medtronic Diabetes has a host of online educational support that you can access at: http://www.medtronicdiabetes.com/infusion-set-support.
IMPORTANT REMINDER: If you notice insulin continuing to drip or squirt from the infusion set cannula when priming/fill-tubing has been completed, do not insert and refer to the full instructions below before starting over with a new reservoir and set.
Please check that you’ve entered your REF and LOT numbers correctly. Otherwise, this may happen for one of four reasons:
| Do Not Use: | Use: |
|---|---|
1) Expired Infusion Sets |
2) Medtronic infusion sets used with a non-Medtronic pump (with a luer lock connector)
3) Infusion sets without tubing (MMT-369, MMT-370, MMT-870, MMT-860) 4) New and enhanced infusion sets received within last two weeks |